| General Information of Drug (ID:
DR3504) |
| Drug Name |
Mobocertinib
|
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; TAK788; AP32788; AP-32788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
|
| Indication |
Lung cancer
[ICD11: 2C25]
|
Phase 3
|
[1]
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
585.7 |
Topological Polar Surface Area |
114 |
| Heavy Atom Count |
43 |
Rotatable Bond Count |
13 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
9 |
| Cross-matching ID |
- PubChem CID
- 118607832
- CAS Number
-
- TTD Drug ID
- DI4HA7
- Formula
- C32H39N7O4
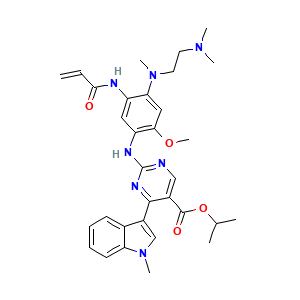
- Canonical SMILES
- CC(C)OC(=O)C1=CN=C(N=C1C2=CN(C3=CC=CC=C32)C)NC4=C(C=C(C(=C4)NC(=O)C=C)N(C)CCN(C)C)OC
- InChI
- InChI=1S/C32H39N7O4/c1-9-29(40)34-24-16-25(28(42-8)17-27(24)38(6)15-14-37(4)5)35-32-33-18-22(31(41)43-20(2)3)30(36-32)23-19-39(7)26-13-11-10-12-21(23)26/h9-13,16-20H,1,14-15H2,2-8H3,(H,34,40)(H,33,35,36)
- InChIKey
- AZSRSNUQCUDCGG-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


