| General Information of Drug (ID:
DR4945) |
| Drug Name |
sparsentan
|
| Synonyms |
Sparsentan; RE-021; 254740-64-2; UNII-9242RO5URM; PS433540; PS-433540; CHEMBL539423; 9242RO5URM; BMS-346567; retrophin; Sparsentan [USAN]; compound 7 [PMID 15634011]; PS 33540; Sparsentan (RE-021); Sparsentan(PS433540); SCHEMBL535109; GTPL8448; BCP23969; BDBM50175523; SB16876; DB12548; CS-7947; DARA-a (Dual Acting Receptor Antagonist of angiotension and endothelin receptors); HY-17621; L023324; 4'-((2-butyl-4-oxo-1,3-diazaspiro[44]non-1-en-3-yl)methyl)-N-(4,5-dimethylisoxazol-3-yl)-2'-(ethoxymethyl)-[1,1'-biphenyl]-2-sulfonamide; RE-021
|
| Indication |
Focal segmental glomerulosclerosis
[ICD11: MF8Y]
|
Phase 3
|
[1]
|
|
Hypertension
[ICD11:
ICD11: BA00-BA04]
|
Phase 2
|
[2]
|
|
Myocardial infarction
[ICD11:
ICD11: BA41-BA43]
|
Phase 2
|
[3]
|
| Structure |
|
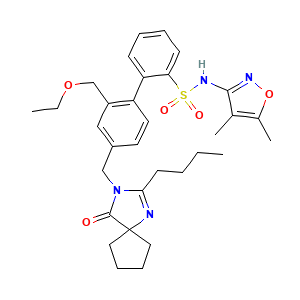 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
592.8 |
Topological Polar Surface Area |
123 |
| Heavy Atom Count |
42 |
Rotatable Bond Count |
12 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 10257882
- CAS Number
-
- TTD Drug ID
- D0YS1U
- Formula
- C32H40N4O5S
- Canonical SMILES
- CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC(=C(C=C3)C4=CC=CC=C4S(=O)(=O)NC5=NOC(=C5C)C)COCC
- InChI
- InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35)
- InChIKey
- WRFHGDPIDHPWIQ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


