| Synonyms |
Valstar; Valrubicin [USAN]; Valstar Preservative Free; AD 32; Antibiotic AD 32; Valstar (TN); N-Trifluoroacetyladriamycin 14-valerate; N-Trifluoroacetyldoxorubicin 14-valerate; Trifluoroacetyladriamycin-14-valerate; Valrubicin (USP/INN); N-Trifluoroacetyladriamycin-14-valerate; Adriamycin, trifluoroacetyl-, 14-valerate; [2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-[5-hydroxy-6-methyl-4-[(2,2,2-trifluoroacetyl)amino]oxan-2-yl]oxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl] pentanoate; (2S-cis)-2-(1,2,3,4,6,11-Hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphthacenyl)-2-oxoethyl pentanoate; (2S-cis)-Pentanoic acid, 2-(1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphth acenyl)-2-oxoethyl ester; (8S,10S)-8-Glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-((2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)-alpha-L-lyxo-hexopyranosyl)oxy)-5,12-naphthacenedione 8(sup 2)-valerate; Pentanoic acid, 2-((2S,4S)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetylamino)-, alpha-L-lysohexopyranoxyl)oxy)-2-naphthacenyl)-2-oxoethyl ester
|
| Cross-matching ID |
- PubChem CID
- 454216
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07IPB
- Formula
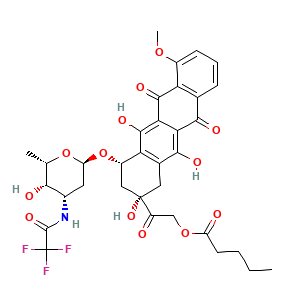
- C34H36F3NO13
- Canonical SMILES
- CCCCC(=O)OCC(=O)[C@]1(C[C@@H](C2=C(C1)C(=C3C(=C2O)C(=O)C4=C(C3=O)C=CC=C4OC)O)O[C@H]5C[C@@H]([C@@H]([C@@H](O5)C)O)NC(=O)C(F)(F)F)O
- InChI
- InChI=1S/C34H36F3NO13/c1-4-5-9-21(40)49-13-20(39)33(47)11-16-24(19(12-33)51-22-10-17(27(41)14(2)50-22)38-32(46)34(35,36)37)31(45)26-25(29(16)43)28(42)15-7-6-8-18(48-3)23(15)30(26)44/h6-8,14,17,19,22,27,41,43,45,47H,4-5,9-13H2,1-3H3,(H,38,46)/t14-,17-,19-,22-,27+,33-/m0/s1
- InChIKey
- ZOCKGBMQLCSHFP-KQRAQHLDSA-N
|