| Synonyms |
D-Penicillamine; penicillamine; 52-67-5; Cuprimine; D-(-)-Penicillamine; 3-Mercapto-D-valine; Depen; Cuprenil; D-Penamine; (-)-Penicillamine; (2S)-2-Amino-3-methyl-3-sulfanylbutanoic acid; D-Mercaptovaline; Mercaptovaline; Perdolat; Penicillamin; Pendramine; Kuprenil; Depamine; Mercaptyl; Trolovol; Metalcaptase; Artamine; Cupripen; (S)-3,3-Dimethylcysteine; D-Valine, 3-mercapto-; Penicillaminum; Penicilamina; Sufirtan; beta-Thiovaline; Dimethylcysteine; D-beta,beta-Dimethylcysteine; D-3-Mercaptovaline; beta,beta-Dimethylcysteine; Penicillamina; Penicilllamine; Sufortan; Copper penicillaminate; D Penicillamine; Penicillamina [DCIT]; Reduced penicillamine; D 3 Mercaptovaline; TBB068824; Beta,beta Dimethylcysteine; Beta-Thiovaline; Cuprimine (TN); D-Penicilamine; D-Penicyllamine; Depen (TN); P-1280; Penicilamina [INN-Spanish]; Penicillaminate, Copper; Penicillaminum [INN-Latin]; Reduced D-penicillamine; Beta,beta-Dimethylcysteine; D,3-Mercaptovaline; D-beta-Mercaptovaline; Distamine (*Hydrochloride*); Metalcaptase (*Hydrochloride*); Penicillamine (JAN/USP/INN); Penicillamine [USAN:INN:BAN:JAN]; Alpha-Amino-beta-methyl-beta-mercaptobutyric acid; D-(-)-2-Amino-3-mercapto-3-methylbutanoic acid; (2S)-2-amino-3-methyl-3-sulfanyl-butanoic acid; (D)-PENICILLAMINE; (S)-Penicillamin; (S)-Penicillamine; 2-Amino-3-mercapto-3-methylbutanoic acid; 3,3-Dimethyl-D-cysteine; 3-Mercaptovaline; 3-sulfanyl-D-valine; D-penicillamine
|
| Cross-matching ID |
- PubChem CID
- 5852
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08HZC
- Formula
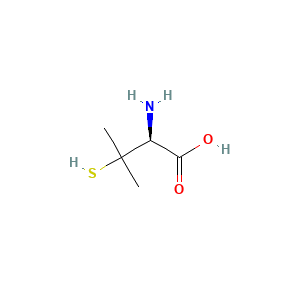
- C5H11NO2S
- Canonical SMILES
- CC(C)([C@H](C(=O)O)N)S
- InChI
- InChI=1S/C5H11NO2S/c1-5(2,9)3(6)4(7)8/h3,9H,6H2,1-2H3,(H,7,8)/t3-/m0/s1
- InChIKey
- VVNCNSJFMMFHPL-VKHMYHEASA-N
|