| Synonyms |
Biphenabid; Bisbid; Bisphenabid; Lesterol; Lorelco; Lursell; Lurselle; Panavir; Probucolum; Sinlestal; Superlipid; Almirall Brand of Probucol; Aventis Brand of Probucol; Hoechst Brand of Probucol; DH 581; DH581; DE-3872; DH-581; LORELCO (TN); Probucolum [INN-Latin]; ZERO/001429; Probucol (JAN/USP/INN); Probucol [USAN:BAN:INN:JAN]; Acetone bis(3,5-di-tert-butyl-4-hydroxyphenyl) mercaptole; Acetone, bis (3,5-di-tert-butyl-4-hydroxyphenyl) mercaptole; Acetone, bis(3,5-di-tert-butyl-4-hydroxyphenyl) mercaptole; 2,6-ditert-butyl-4-[2-(3,5-ditert-butyl-4-hydroxyphenyl)sulfanylpropan-2-ylsulfanyl]phenol; 4,4'-(Isopropylidenedithio)bis(2, 6-di-tert-butylphenol); 4,4'-(Isopropylidenedithio)bis(2,6-di-tert-butylphenol); 4,4'-(Isopropylidenedithio)bis[2, 6-di-tert-butylphenol]; 4,4'-(Isopropylidenedithio)bis[2,6-di-tert-butylphenol]; 4,4'-(propane-2,2-diyldisulfanediyl)bis(2,6-di-tert-butylphenol); 4,4'-[(1-Methylethylidene)bis(thio)]bis-[2,6-bis(1,1-dimethylethyl)phenol]
|
| Cross-matching ID |
- PubChem CID
- 4912
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0H2DQ
- Formula
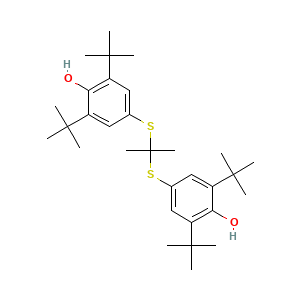
- C31H48O2S2
- Canonical SMILES
- CC(C)(C)C1=CC(=CC(=C1O)C(C)(C)C)SC(C)(C)SC2=CC(=C(C(=C2)C(C)(C)C)O)C(C)(C)C
- InChI
- InChI=1S/C31H48O2S2/c1-27(2,3)21-15-19(16-22(25(21)32)28(4,5)6)34-31(13,14)35-20-17-23(29(7,8)9)26(33)24(18-20)30(10,11)12/h15-18,32-33H,1-14H3
- InChIKey
- FYPMFJGVHOHGLL-UHFFFAOYSA-N
|