Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR5582) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
NERATINIB MALEATE
|
|||||
| Synonyms | Neratinib maleate; UNII-9RM7XY23ZS; 9RM7XY23ZS; Neratinib maleate [MI]; hki-272 maleate; Nerlynx (TN); SCHEMBL2180998; MolPort-044-561-003; AKOS030524209; DS-19892 | |||||
| Indication | HER2/NEU overexpressing breast cancer [ICD11: 2C60-2C6Y] | Approved | [1] | |||
| Structure |
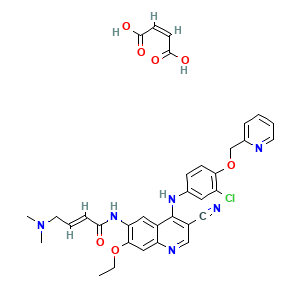 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 673.1 | Topological Polar Surface Area | 187 | ||
| Heavy Atom Count | 48 | Rotatable Bond Count | 13 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 12 | |||
| Cross-matching ID |
|
|||||
| The Predicted Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Predicted Drug Metabolites (PDM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| 2 | DrugBank(Pharmacology-Metabolism):NERATINIB MALEATE | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

