| Synonyms |
Corvert; Ibutilida; Ibutilidum; Butilide fumarate; IBUTILIDE FUMARATE; Ibutilide Fumarate [USAN]; U 70226E; U82208E; U82209E; Corvert (TN); Ibutilida [INN-Spanish]; Ibutilide (INN); Ibutilide [BAN:INN]; Ibutilide [INN:BAN]; Ibutilide fumarate (USAN); Ibutilidum [INN-Latin]; U-70226E; U-82208E; U-82209E; Ibutilide, (+-)-isomer; N-[4-[4-[ethyl(heptyl)amino]-1-hydroxybutyl]phenyl]methanesulfonamide; Ibutilide, fumarate salt (2:1), (+-)-isomer; N-(4-(4-(ethylheptylamino)-1-hydroxybutyl)phenyl)methanesulfonamide; N-(4-{4-[ethyl(heptyl)amino]-1-hydroxybutyl}phenyl)methanesulfonamide; Methanesulfonamide, N-(4-(4-(ethylheptylamino)-1-hydroxybutyl)phenyl)-, (E)-2-butenedioate (2:1) (salt); Methanesulfonamide, N-(4-(4-(ethylheptylamino)-1-hydroxybutyl)phenyl)-, (+-)-, (E)-2-butenedioate (2:1) (salt); Methanesulfonamide, N-(4-(4-(ethylheptylamino)-1-hydroxybutyl)phenyl)-, (+-)-,(E)-2-butenedioate (2:1) (salt); (+-)-4'-(4-(Ethylheptylamino)-1-hydroxybutyl)methanesulfoanilide (E)-2-butenedioate (2:1); (+-)-4'-(4-(Ethylheptylamino)-1-hydroxybutyl)methanesulfonanilide fumarate (2:1) (salt); (+-)-N-(4-(4-(Ethylheptylamino)-1-hydroxybutyl)phenyl)methanesulfonamide (E)-butenedioate
|
| Cross-matching ID |
- PubChem CID
- 60753
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02MLW
- Formula
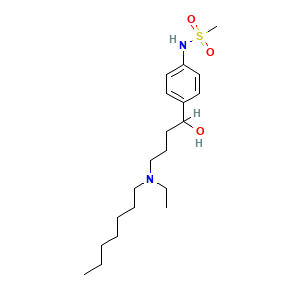
- C20H36N2O3S
- Canonical SMILES
- CCCCCCCN(CC)CCCC(C1=CC=C(C=C1)NS(=O)(=O)C)O
- InChI
- InChI=1S/C20H36N2O3S/c1-4-6-7-8-9-16-22(5-2)17-10-11-20(23)18-12-14-19(15-13-18)21-26(3,24)25/h12-15,20-21,23H,4-11,16-17H2,1-3H3
- InChIKey
- ALOBUEHUHMBRLE-UHFFFAOYSA-N
|