| General Information of This Metabolic Reaction (MR) (ID:
MR001640) |
| Formula |
|
| Reactant |
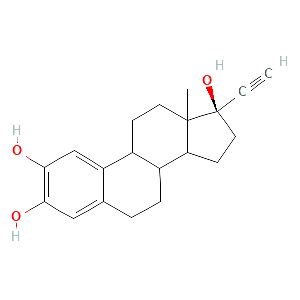
2-hydroxyethinylestradiol |
Product |
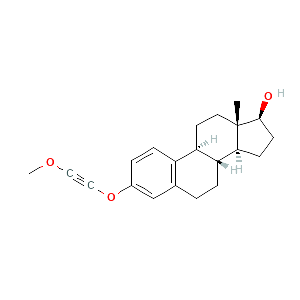
2-methoxyethinylestradiol |
|
Reactant Info
|
Product Info
|
|
Metabolic Enzyme
|
Monoamine oxidase type A (MAO-A)
|
DME Info
|
|
Metabolic Type
|
Conjugation
-
O-Methylation
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Reactant of This MR |
|
Other MR(s) That Produce The Reactant of This MR
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Produce The Product of This MR
|
|
|
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
Sulfotransferase 1E1 is a low km isoform mediating the 3-O-sulfation of ethinyl estradiol Drug Metab Dispos. 2004 Nov;32(11):1299-303. doi: 10.1124/dmd.32.11..
|
| 2 |
Genetic variation in the first-pass metabolism of ethinylestradiol, sex hormone binding globulin levels and venous thrombosis risk Eur J Intern Med. 2017 Jul;42:54-60. doi: 10.1016/j.ejim.2017.05.019.
|
| 3 |
Metabolism of 17 alpha-ethinylestradiol by human liver microsomes in vitro: aromatic hydroxylation and irreversible protein binding of metabolites J Clin Endocrinol Metab. 1974 Dec;39(6):1072-80. doi: 10.1210/jcem-39-6-1072.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.