| References |
| 1 |
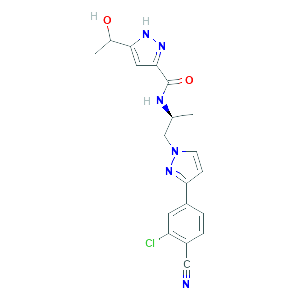
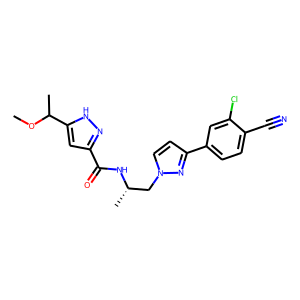
Clinical Development of Darolutamide: A Novel Androgen Receptor Antagonist for the Treatment of Prostate Cancer Clin Genitourin Cancer. 2018 Oct;16(5):332-340. doi: 10.1016/j.clgc.2018.07.017.
|
| 2 |
Drug-Drug Interaction Study to Evaluate the Pharmacokinetics, Safety, and Tolerability of Ipatasertib in Combination with Darolutamide in Patients with Advanced Prostate Cancer. Pharmaceutics. 2022 Oct 1;14(10):2101. doi: 10.3390/pharmaceutics14102101.
|
| 3 |
Clinical Pharmacokinetics of the Androgen Receptor Inhibitor Darolutamide in Healthy Subjects and Patients with Hepatic or Renal Impairment. Clin Pharmacokinet. 2022 Apr;61(4):565-575. doi: 10.1007/s40262-021-01078-y.
|
| 4 |
Metabolism and Mass Balance of the Novel Nonsteroidal Androgen Receptor Inhibitor Darolutamide in Humans Drug Metab Dispos. 2021 Jun;49(6):420-433. doi: 10.1124/dmd.120.000309.
|