Details of the Metabolic Reaction (MR)
| General Information of This Metabolic Reaction (MR) (ID: MR009118) | ||||||
|---|---|---|---|---|---|---|
| Formula |
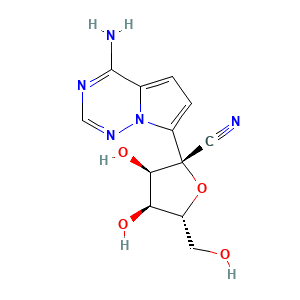 Unclear
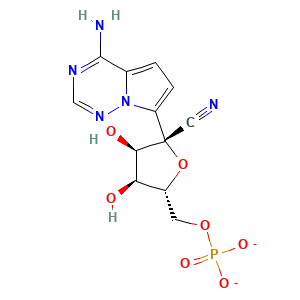 |
|||||
| Reactant | GS-441524 | Product | GS-441524-MP | |||
| Reactant Info | Product Info | |||||
| Metabolic Type | Unclear - Unclear | |||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir | |||||
| 2 | Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19 | |||||
| 3 | Comparison of anti-SARS-CoV-2 activity and intracellular metabolism of remdesivir and its parent nucleoside | |||||
| 4 | Population pharmacokinetic modeling of GS-441524, the active metabolite of remdesivir, in Japanese COVID-19 patients with renal dysfunction | |||||
| 5 | Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19 | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

