| General Information of Drug Metabolite (DM) (ID: DM000567) |
| DM Name |
Efavirenz metabolite M1
|
| Structure |
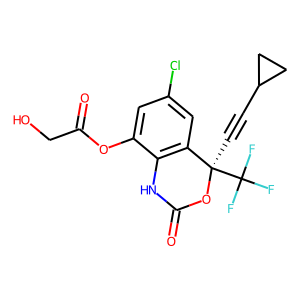
|
|
3D MOL is unavailable
|
2D MOL is unavailable
|
|
|
|
|
|
|
|
| Full List of Drug-Metabolizing Enzyme (DME) Related to This DM |
| DME(s) Producing This DM through Metabolism |
| DME Name |
DME ID |
Reactant |
Reaction |
Related Drug |
REF |
|
UDP-glucuronyltransferase (UGT)
|
DMEN064
|
| Conjugation - Conjugation |
Efavirenz
|
[1] , [2] |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Full List of Drug(s) That Produce This DM By Metabolism |
| Efavirenz |
DR0560
|
Approved |
Human immunodeficiency virus infection |
|
|
| References |
| 1 |
Clinical pharmacokinetics and drug-drug interactions of Elbasvir/Grazoprevir. Eur J Drug Metab Pharmacokinet. 2018 Oct;43(5):509-531.
|
| 2 |
The Activity of Members of the UDP-Glucuronosyltransferase Subfamilies UGT1A and UGT2B is Impaired in Patients with Liver Cirrhosis. Clin Pharmacokinet. 2023 Jun 16. doi: 10.1007/s40262-023-01261-3.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


