Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0012) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Benzotetronic acid
|
|||||
| Synonyms |
Coumarin, 4-hydroxy-; X954ZLL2RD; 1076-38-6; 2-Hydroxychromone; 2-hydroxychromen-4-one; 2H-1-BENZOPYRAN-2-ONE, 4-HYDROXY-; 4-Coumarinol; 4-Hydroxycoumarin; 4-HYDROXY-1-BENZOPYRAN-2-ONE; 4-Hydroxy-2H-1-benzopyran-2-one; 4-Hydroxy-2H-chromen-2-one; 4-Hydroxy-chromen-2-one; 4-Hydroxycoumarin, 98%; 4-Hydroxycoumarine; 4-hydroxy-2-chromenone; 4-hydroxy-coumarin; 4-hydroxychromen-2-one; 4H-1-Benzopyran-4-one, 2-hydroxy-; AI3-52393; BRN 0129768; CHEMBL301141; EINECS 214-060-2; MFCD00006856; NSC 11889; UNII-X954ZLL2RD
|
|||||
| Indication | Discovery agent | Investigative | [1] | |||
| Structure |
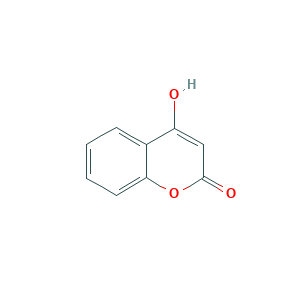 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 162.14 | Topological Polar Surface Area | 46.5 | ||
| Heavy Atom Count | 12 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

