| Cross-matching ID |
- PubChem CID
- 1978
- PubChem SID
-
9022
; 5199954
; 7849397
; 7978630
; 8148212
; 8151358
; 10520758
; 11335787
; 11361026
; 11363385
; 11365947
; 11368509
; 11371586
; 11374422
; 11376671
; 11461998
; 11466097
; 11467217
; 11485235
; 11485914
; 11489156
; 11490389
; 11492617
; 11494305
; 15075320
; 29221166
; 46509113
; 47216767
; 47216768
; 47291119
; 47365171
; 47365172
; 47440234
; 47810742
; 49698901
; 49895324
; 50427938
; 56413043
; 57321077
; 85209697
; 85787903
; 99222788
; 103189011
; 104299258
; 124883487
; 126443068
; 126679412
; 127982055
; 134337777
; 134999047
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0HD9K
- Formula
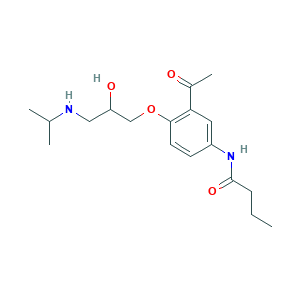
- C18H28N2O4
- Canonical SMILES
- CCCC(=O)NC1=CC(=C(C=C1)OCC(CNC(C)C)O)C(=O)C
- InChI
- 1S/C18H28N2O4/c1-5-6-18(23)20-14-7-8-17(16(9-14)13(4)21)24-11-15(22)10-19-12(2)3/h7-9,12,15,19,22H,5-6,10-11H2,1-4H3,(H,20,23)
- InChIKey
- GOEMGAFJFRBGGG-UHFFFAOYSA-N
|