| Synonyms |
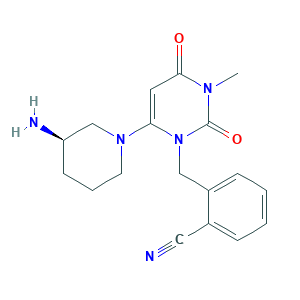
Alogliptin; Alogliptin (SYR-322); Alogliptin [INN]; JHC049LO86; alogliptina; alogliptine; alogliptinum; vipidia; (R)-2-((6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)benzonitrile; 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl}methyl)benzonitrile; 2-({6-[(3r)-3-Aminopiperidin-1-Yl]-3-Methyl-2,4-Dioxo-3,4-Dihydropyrimidin-1(2h)-Yl}methyl)benzonitrile; 850649-61-5; AK322010; CHEBI:72323; HSDB 8203; UNII-JHC049LO86
|
| Cross-matching ID |
- PubChem CID
- 11450633
- PubChem SID
-
16549582
; 23591487
; 42529629
; 46516529
; 57376680
; 79634689
; 87544227
; 93300285
; 103516453
; 126665588
; 134339050
; 135207592
; 137147468
; 140812881
; 160962873
; 162009811
; 162253849
; 164193948
; 164835224
; 175265639
; 175427056
; 178102937
; 185988964
; 210279140
; 210281462
; 223574691
; 223683116
; 224085697
; 226492557
; 241154940
; 244648029
; 249810610
; 251971115
; 252214702
; 252553765
; 252671739
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0NJ5H
- Formula
- C18H21N5O2
- Canonical SMILES
- CN1C(=O)C=C(N(C1=O)CC2=CC=CC=C2C#N)N3CCCC(C3)N
- InChI
- 1S/C18H21N5O2/c1-21-17(24)9-16(22-8-4-7-15(20)12-22)23(18(21)25)11-14-6-3-2-5-13(14)10-19/h2-3,5-6,9,15H,4,7-8,11-12,20H2,1H3/t15-/m1/s1
- InChIKey
- ZSBOMTDTBDDKMP-OAHLLOKOSA-N
|