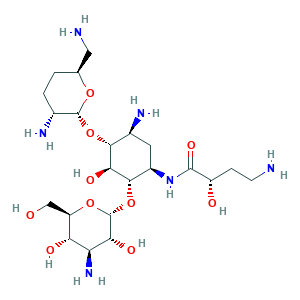
| Synonyms |
Arbekacin; Arbekacin (INN); Arbekacin [INN]; Arbekacina; Arbekacina [Spanish]; Arbekacine; Arbekacine [French]; Arbekacinum; Arbekacinum [Latin]; Haberacin; NPC-14; 51025-85-5; CHEBI:37922; G7V6SLI20L; O-3-Amino-3-deoxy-alpha-D-glucopyranosyl-(1-4)-O-(2,6-diamino-2,3,4,6-tetradeoxy-alpha-D-erythro-hexopyranosyl-(1-6))-N'-((2S)-4-amino-2-hydroxybutyryl)-2-deoxy-L-streptamine; UNII-G7V6SLI20L
|
| Cross-matching ID |
- PubChem CID
- 68682
- PubChem SID
-
8192273
; 12012746
; 15012131
; 24434905
; 43125217
; 50047867
; 50112783
; 50764410
; 51091793
; 57317006
; 99443250
; 103027235
; 103069356
; 103080181
; 103508341
; 104342778
; 117570307
; 124766131
; 126671210
; 134223846
; 135027886
; 136290748
; 137248758
; 164788050
; 178103917
; 179150519
; 184544961
; 198976904
; 226408426
; 241090282
; 252358771
; 252671973
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07JPC
- Formula
- C22H44N6O10
- Canonical SMILES
- C1CC(C(OC1CN)OC2C(CC(C(C2O)OC3C(C(C(C(O3)CO)O)N)O)NC(=O)C(CCN)O)N)N
- InChI
- 1S/C22H44N6O10/c23-4-3-12(30)20(34)28-11-5-10(26)18(37-21-9(25)2-1-8(6-24)35-21)17(33)19(11)38-22-16(32)14(27)15(31)13(7-29)36-22/h8-19,21-22,29-33H,1-7,23-27H2,(H,28,34)/t8-,9+,10-,11+,12-,13+,14-,15+,16+,17-,18+,19-,21+,22+/m0/s1
- InChIKey
- MKKYBZZTJQGVCD-XTCKQBCOSA-N
|