| General Information of Drug (ID:
DR0478) |
| Drug Name |
Dextropropoxyphene
|
| Synonyms |
Destropropossifene; Destropropossifene [DCIT]; Dextropropoxifeno; Dextropropoxifeno [INN-Spanish]; Dextropropoxyphen; Dextropropoxyphene; Dextropropoxyphene [INN:BAN]; Dextropropoxyphenum; Dextropropoxyphenum [INN-Latin]; Dextroproxifeno; Dextroproxifeno [Spanish]; Femadol; Propoxyphene, (+)-; Proxagesic; d-Propoxyphene; Algafan; Antalvic; Darvon; propoxyphene; (+)-1,2-Diphenyl-2-propionoxy-3-methyl-4-dimethylaminobutane; (D)-PROPOXYPHENE; 4-Dimethylamino-3-methyl-1,2-diphenyl-2-propoxybutane; 469-62-5; SK 65; UNII-S2F83W92TK
|
| Indication |
Lung cancer
[ICD11: 2C25]
|
Phase 4
|
[1]
|
| Structure |
|
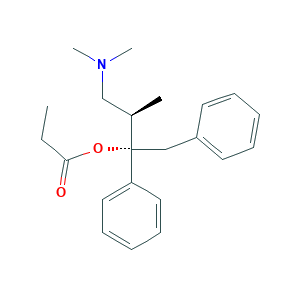 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
339.5 |
Topological Polar Surface Area |
29.5 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 10100
- PubChem SID
-
9610
; 7979056
; 8157284
; 15025964
; 29228634
; 46506690
; 48416483
; 49896450
; 53790711
; 56464340
; 57325958
; 74390898
; 96024506
; 104239521
; 104322376
; 134337347
; 134975162
; 136279476
; 136350241
; 137003058
; 139157528
; 162221259
; 175267938
; 176267062
; 179116862
; 198954437
; 223365928
; 226413489
; 241078575
; 250138664
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D4PB
- Formula
- C22H29NO2
- Canonical SMILES
- CCC(=O)OC(CC1=CC=CC=C1)(C2=CC=CC=C2)C(C)CN(C)C
- InChI
- 1S/C22H29NO2/c1-5-21(24)25-22(18(2)17-23(3)4,20-14-10-7-11-15-20)16-19-12-8-6-9-13-19/h6-15,18H,5,16-17H2,1-4H3/t18-,22+/m1/s1
- InChIKey
- XLMALTXPSGQGBX-GCJKJVERSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


