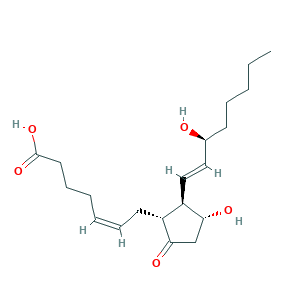
| Synonyms |
Dinoproston; Dinoprostona; Dinoprostona [INN-Spanish]; Dinoprostone; Dinoprostonum; Dinoprostonum [INN-Latin]; Minprositin E2; Minprostin E2; Cervidil; PGE2alpha; Prepidil; Propess; Prostaglandin E; Prostaglandin E2; Prostaglandin E2alpha; Prostarmon E; Prostin; Prostin E2; U-12062; l-PGE2; l-Prostaglandin E2; (15S)-Prostaglandin E2; (5Z,11alpha,13E,15S)-11,15-dihydroxy-9-oxoprosta-5,13-dien-1-oic acid; 363-24-6; NSC 165560; PGE2; UNII-K7Q1JQR04M; [3H]PGE2
|
| Cross-matching ID |
- PubChem CID
- 5280360
- PubChem SID
-
3863
; 439749
; 450259
; 599053
; 803734
; 3139923
; 3727087
; 4265954
; 7847147
; 7979101
; 8143217
; 8616235
; 10321741
; 14778621
; 14900948
; 24898100
; 24898683
; 24898775
; 26753268
; 26753269
; 26753270
; 26759401
; 39289526
; 46505549
; 47662021
; 47810515
; 47885164
; 48415907
; 50026789
; 50087222
; 50105678
; 50105679
; 53789659
; 56313306
; 56314112
; 56459056
; 57357707
; 75054349
; 85789494
; 85856640
; 92298396
; 92308753
; 92309906
; 92722489
; 99300820
; 99302338
; 99431525
; 103176817
; 104017641
; 104046436
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06FEA
- Formula
- C20H32O5
- Canonical SMILES
- CCCCCC(C=CC1C(CC(=O)C1CC=CCCCC(=O)O)O)O
- InChI
- 1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1
- InChIKey
- XEYBRNLFEZDVAW-ARSRFYASSA-N
|