| Synonyms |
Docetaxel anhydrous; Docetaxel, 98%; Docetaxel, Trihydrate; Docetaxol; Docetaxolum [INN-Latin]; RP 56976; RP-56976; Taxotere; Taxotere (TN); XRP6976; docetaxel; 114977-28-5; 699121PHCA; CHEBI:4672; DSSTox_CID_20464; DSSTox_GSID_40464; DSSTox_RID_79497; EmDOC; MFCD00800737; N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetylpaclitaxel; N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol; N-debenzoyl-N-Boc-10-deacetyl taxol; N-debenzoyl-N-tert-butoxycarbonyl-10-deacetyltaxol; NSC 628503; NSC-628503; NSC628503; TXL; UNII-699121PHCA
|
| Cross-matching ID |
- PubChem CID
- 148124
- PubChem SID
-
13410
; 494081
; 822539
; 833094
; 7890914
; 7979114
; 10249812
; 12014196
; 14791415
; 14815974
; 17424977
; 24845209
; 26683886
; 46225079
; 46506766
; 53790583
; 56310805
; 56310954
; 56311107
; 56311313
; 56311574
; 56312625
; 56312880
; 56312940
; 56312941
; 56313207
; 56313989
; 56314033
; 56314070
; 56314178
; 56314280
; 57346703
; 78743198
; 81044598
; 87678200
; 92308884
; 92711403
; 96024562
; 99319097
; 103172104
; 104418276
; 117664410
; 117682515
; 124950708
; 126606827
; 126630894
; 126657365
; 134337881
; 136340291
; 136375539
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O5WP
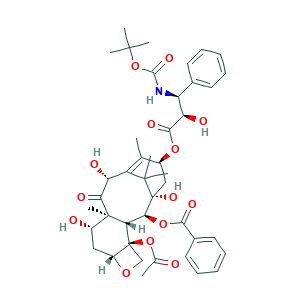
- Formula
- C43H53NO14
- Canonical SMILES
- CC1=C2C(C(=O)C3(C(CC4C(C3C(C(C2(C)C)(CC1OC(=O)C(C(C5=CC=CC=C5)NC(=O)OC(C)(C)C)O)O)OC(=O)C6=CC=CC=C6)(CO4)OC(=O)C)O)C)O
- InChI
- 1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1
- InChIKey
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N
|