| General Information of Drug (ID:
DR0522) |
| Drug Name |
Dofetilide
|
| Synonyms |
Dofetilida; Dofetilida [INN-Spanish]; Dofetilide; Dofetilide (Tikosyn); Dofetilide(Tikosyn); Dofetilidum; Dofetilidum [INN-Latin]; R4Z9X1N2ND; Tikosyn; UK 68,798; UK 68798; UK-68,798; UK-68798; 1-(4-Methanesulphonamidophenoxy)-2-[N-(4-methanesulphonamidophenethyl)-N-methylamino]ethane; 115256-11-6; C19H27N3O5S2; CHEBI:4681; CHEMBL473; N-[4-[2-[Methyl[2-[4-[(methylsulfonyl)amino]phenoxy]ethyl]amino]ethyl]phenyl]methanesulfonamide; UNII-R4Z9X1N2ND; beta-((p-Methanesulfonamidophenethyl)methylamino)methanesulfono-p-phenetidide
|
| Indication |
Atrial fibrillation
[ICD11: BC81]
|
Approved
|
[1]
|
| Structure |
|
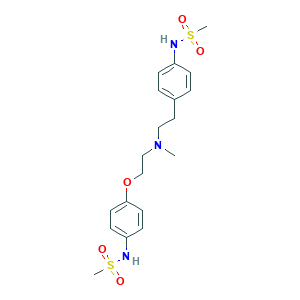 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
441.6 |
Topological Polar Surface Area |
122 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
11 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 71329
- PubChem SID
-
9953
; 6866968
; 7847713
; 7979117
; 8194566
; 11528674
; 12014163
; 14832836
; 26719840
; 26757968
; 43127674
; 46386638
; 46509127
; 49681772
; 49830467
; 50139453
; 50845418
; 57318136
; 81041120
; 87350381
; 92308504
; 92308779
; 92719410
; 103168841
; 103940282
; 104253292
; 104350198
; 117632008
; 119526323
; 121361247
; 124659024
; 124757413
; 124800159
; 125001905
; 125164217
; 125338634
; 126620831
; 126658284
; 126670949
; 128235810
; 131295440
; 131542548
; 134337788
; 135028787
; 135650201
; 135692215
; 136946393
; 137005478
; 140086817
; 144115915
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0NW3X
- Formula
- C19H27N3O5S2
- Canonical SMILES
- CN(CCC1=CC=C(C=C1)NS(=O)(=O)C)CCOC2=CC=C(C=C2)NS(=O)(=O)C
- InChI
- 1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3
- InChIKey
- IXTMWRCNAAVVAI-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


