| Synonyms |
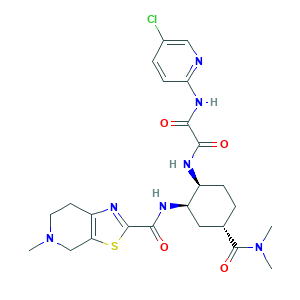
Edoxaban (USAN/INN); Edoxaban [USAN:INN]; Edoxaban hydrochloride; HGVDHZBSSITLCT-JLJPHGGASA-N; Lixiana; NDU3J18APO; PB31142; DU-176; DU-176b; EDOXABAN; SAVAYSA; SCHEMBL330046; ZINC43200832; 480449-70-5; 912273-65-5; ACN-039922; AKOS005146069; AOB87348; BDBM50328731; C24H30ClN7O4S; CHEBI:85973; CHEMBL1269025; DTXSID50197398; GTPL7575; KS-000004VY; MFCD13195544; N1-(5-chloropyridin-2-yl)-N2-((1S,2R,4S)-4-(dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl)oxalamide; UNII-NDU3J18APO
|
| Cross-matching ID |
- PubChem CID
- 10280735
- PubChem SID
-
15285745
; 22657909
; 35552742
; 75383420
; 109693269
; 123092995
; 123107846
; 124490450
; 135263753
; 141477305
; 152343994
; 162198449
; 163409416
; 163620696
; 163686010
; 163909302
; 170502825
; 172096222
; 172121849
; 185988955
; 198981897
; 198992538
; 223365911
; 223397933
; 223922733
; 226666786
; 249273064
; 249736672
; 249865853
; 252090008
; 252150280
; 252451702
; 252552142
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C3BS
- Formula
- C24H30ClN7O4S
- Canonical SMILES
- CN1CCC2=C(C1)SC(=N2)C(=O)NC3CC(CCC3NC(=O)C(=O)NC4=NC=C(C=C4)Cl)C(=O)N(C)C
- InChI
- 1S/C24H30ClN7O4S/c1-31(2)24(36)13-4-6-15(27-20(33)21(34)30-19-7-5-14(25)11-26-19)17(10-13)28-22(35)23-29-16-8-9-32(3)12-18(16)37-23/h5,7,11,13,15,17H,4,6,8-10,12H2,1-3H3,(H,27,33)(H,28,35)(H,26,30,34)/t13-,15-,17+/m0/s1
- InChIKey
- HGVDHZBSSITLCT-JLJPHGGASA-N
|