| Synonyms |
Encorafenib; Encorafenib (JAN/USAN/INN); Encorafenib (LGX818); Encorafenib [USAN:INN]; Braftovi; Braftovi (TN); CMJCXYNUCSMDBY-ZDUSSCGKSA-N; LGX 818; LGX-818; LGX-818(Encorafenib); LGX818; NVP-LGX818; NVP-LGX818-NXA; SCHEMBL8228295; 1269440-17-6; 8L7891MRB6; BCP08458; BDBM221688; CHEMBL3301612; Carbamic acid, N-[(1S)-2-[[4-[3-[5-chloro-2-fluoro-3-[(methylsulfonyl)amino]phenyl]-1-(1-methylethyl)-1H-pyrazol-4-yl]-2-pyrimidinyl]amino]-1-methylethyl]-, methyl ester; DTXSID00155347; EX-A1587; GTPL7908; UNII-8L7891MRB6
|
| Cross-matching ID |
- PubChem CID
- 50922675
- PubChem SID
-
113916820
; 138935331
; 163643304
; 172821370
; 177218161
; 184024317
; 184024338
; 184525737
; 184525758
; 184525779
; 198937762
; 210275262
; 210280903
; 224552863
; 226088091
; 233756042
; 242651812
; 249565591
- CAS Number
-
- TTD Drug ID
- D0TK7R
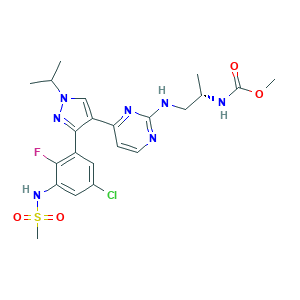
- Formula
- C22H27ClFN7O4S
- Canonical SMILES
- CC(C)N1C=C(C(=N1)C2=CC(=CC(=C2F)NS(=O)(=O)C)Cl)C3=NC(=NC=C3)NCC(C)NC(=O)OC
- InChI
- 1S/C22H27ClFN7O4S/c1-12(2)31-11-16(17-6-7-25-21(28-17)26-10-13(3)27-22(32)35-4)20(29-31)15-8-14(23)9-18(19(15)24)30-36(5,33)34/h6-9,11-13,30H,10H2,1-5H3,(H,27,32)(H,25,26,28)/t13-/m0/s1
- InChIKey
- CMJCXYNUCSMDBY-ZDUSSCGKSA-N
|