Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0579) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Enflurane
|
|||||
| Synonyms |
Efrane; Enflurane [Anaesthetics, volatile]; Enflurano; Enflurano [INN-Spanish]; Enfluranum; Enfluranum [INN-Latin]; Ethrane; Alyrane; Anesthetic 347; Anesthetic Compound No. 347; Compound 347; Methylflurether; OHIO 347; enflurane; 13838-16-9; 2-Chloro-1,1,2-trifluoroethyl difluoromethyl ether; 2-Chloro-1-(difluoromethoxy)-1,1,2-trifluoroethane; BRN 1903921; C 347; CHEBI:4792; EINECS 237-553-4; Ethane, 2-chloro-1-(difluoromethoxy)-1,1,2-trifluoro-; Ether, 2-chloro-1,1,2-trifluoroethyl difluoromethyl; MFCD00069095; NSC-115944
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Approved | [1] | |||
| Structure |
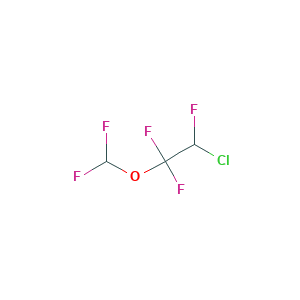 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 184.49 | Topological Polar Surface Area | 9.2 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

