| Synonyms |
Elestat; Epinastina; Epinastina [Spanish]; Epinastine (INN); Epinastine [INN]; Epinastinum; Epinastinum [Latin]; Purivist (TN); Relestat; WAL 80; WAL 801; WAL-80 Cl; epinastine; (+-)-Epinastine; 1H-Dibenz(c,f)imidazo(1,5-a)azepin-3-amino, 9,13b-dihydro-; 3-Amino-9,13b-dihydro-1H-dibenz(c,f)imidazo(1,5-a)azepine; 3-Amino-9,13b-dihydro-1H-dibenz[c,f]imidazo[1,5-a]azepine; 5HDI847257; 79UN26Y71B; 80012-43-7; 9,13b-dihydro-1H-dibenzo[c,f]imidazo[1,5-a]azepin-3-amine; C16H15N3; CHEBI:51032; CHEMBL1106; UNII-5HDI847257; UNII-79UN26Y71B
|
| Cross-matching ID |
- PubChem CID
- 3241
- PubChem SID
-
5173366
; 8152057
; 14847686
; 29217549
; 29222382
; 46509056
; 50065222
; 50111706
; 56464356
; 57321677
; 75376315
; 81041126
; 85209437
; 85286058
; 90340676
; 92714612
; 96024596
; 103305708
; 104022599
; 104302939
; 118313772
; 125357916
; 126629485
; 126655288
; 126684289
; 129584096
; 134338331
; 135026263
; 136912969
; 137101854
; 142087407
; 152034622
; 152253736
; 160964094
; 162176284
; 164814672
; 178103751
; 179148544
; 196106483
; 206246277
; 210279785
; 210282108
; 221672861
; 223381334
; 223435039
; 223681981
; 224278905
; 226408729
; 251912269
; 251916574
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0DV3O
- Formula
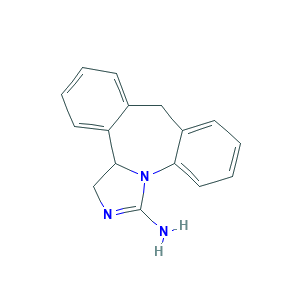
- C16H15N3
- Canonical SMILES
- C1C2C3=CC=CC=C3CC4=CC=CC=C4N2C(=N1)N
- InChI
- 1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18)
- InChIKey
- WHWZLSFABNNENI-UHFFFAOYSA-N
|