| Cross-matching ID |
- PubChem CID
- 41867
- PubChem SID
-
13409
; 793944
; 8177324
; 14716206
; 14812446
; 14837077
; 24769893
; 26704252
; 26710264
; 34707467
; 46507282
; 46530809
; 48415946
; 49995002
; 50070726
; 53787927
; 56311421
; 56313206
; 56313988
; 57288587
; 57288773
; 57312617
; 77126435
; 96024597
; 103164726
; 104338146
; 117597720
; 123080197
; 126686290
; 127301368
; 127301369
; 127301370
; 127301371
; 127301372
; 127301373
; 127301374
; 127301375
; 127301376
; 127301377
; 127301378
; 127301379
; 127301380
; 127301381
; 127301382
; 127301383
; 127301384
; 127301385
; 127301386
; 127301387
; 127301388
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C9XJ
- Formula
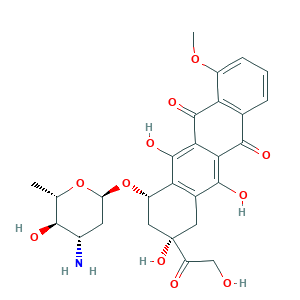
- C27H29NO11
- Canonical SMILES
- CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O
- InChI
- 1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22-,27-/m0/s1
- InChIKey
- AOJJSUZBOXZQNB-VTZDEGQISA-N
|