| General Information of Drug (ID:
DR0837) |
| Drug Name |
Hydroxychloroquine sulfate
|
| Synonyms |
Hydroxychloroquine sulfate [USP]; Hydroxychloroquine sulphate; Plaquinol; TCMDC-123987; Toremonil; 2-((4-((7-Chloroquinolin-4-yl)amino)pentyl)(ethyl)amino)ethanol sulfate; 747-36-4; AI3-52706; AK-72909; DSSTox_CID_27788; DSSTox_RID_82563; EINECS 212-019-3; Ethanol, 2-((4-((7-chloro-4-quinolinyl)amino)pentyl)ethylamino)-, sulfate (1:1) (salt); Ethanol, 2-((4-((7-chloro-4-quinolyl)amino)pentyl)ethylamino)-, sulfate (1:1) (salt); NSC 4375; Ercoquin; HCQ sulfate; HYDROXYCHLOROQUINE SULFATE
|
| Indication |
Malaria
[ICD11: 1F40]
|
Approved
|
[1]
|
| Structure |
|
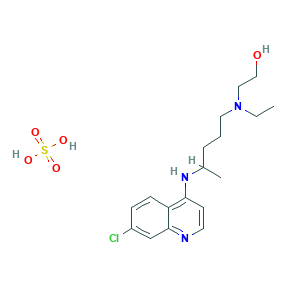 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
434 |
Topological Polar Surface Area |
131 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
8 |
| Cross-matching ID |
- PubChem CID
- 12947
- CAS Number
-
- TTD Drug ID
- D0OJ4L
- Formula
- C18H28ClN3O5S
- Canonical SMILES
- CCN(CCCC(C)NC1=C2C=CC(=CC2=NC=C1)Cl)CCO.OS(=O)(=O)O
- InChI
- 1S/C18H26ClN3O.H2O4S/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18;1-5(2,3)4/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21);(H2,1,2,3,4)
- InChIKey
- JCBIVZZPXRZKTI-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


