| Synonyms |
Imiquimod acetate; Imiquimodum; P1QW714R7M; S 26308; S-26308; Zartra; Zyclara; 1-(2-Methylpropyl)-1H-imidazole[4,5-c]quinoline-4-amine; Aldara; Beselna; IMIQUIMOD; 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine; 1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-amine; 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine; 1-isobutylimidazo[4,5-c]quinolin-4-amine; 4-Amino-1-isobutyl-1H-imidazo(4,5-c)quinoline; 4-Amino-1-isobutyl-1H-imidazo[4,5-c]quinoline; 9050-31-1; 99011-02-6; CHEMBL1282; MFCD00866946; R 837; R-837; UNII-P1QW714R7M
|
| Cross-matching ID |
- PubChem CID
- 57469
- PubChem SID
-
626307
; 865810
; 7740549
; 8185255
; 12013659
; 14749564
; 17396674
; 17425421
; 24724516
; 25623636
; 26752301
; 26758924
; 43115161
; 46505394
; 48426262
; 49835865
; 50065439
; 53788646
; 56313523
; 57313902
; 61128040
; 74724996
; 85174371
; 87561300
; 92309250
; 92712277
; 93167017
; 99437194
; 103396795
; 104098475
; 104312759
; 117524043
; 117779671
; 118046220
; 124757085
; 124800125
; 125163889
; 125325444
; 125360574
; 126592901
; 126630136
; 126656817
; 126670841
; 127765602
; 131331797
; 131789979
; 134337592
; 135015330
; 135698182
; 136023845
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06CTE
- Formula
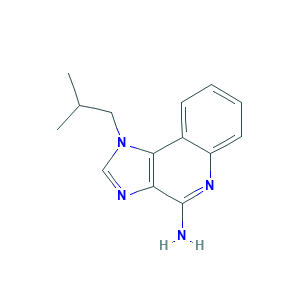
- C14H16N4
- Canonical SMILES
- CC(C)CN1C=NC2=C1C3=CC=CC=C3N=C2N
- InChI
- 1S/C14H16N4/c1-9(2)7-18-8-16-12-13(18)10-5-3-4-6-11(10)17-14(12)15/h3-6,8-9H,7H2,1-2H3,(H2,15,17)
- InChIKey
- DOUYETYNHWVLEO-UHFFFAOYSA-N
|