| Synonyms |
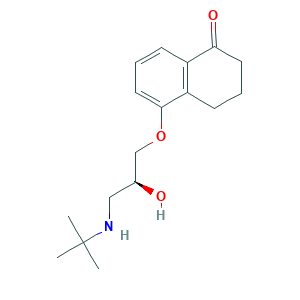
Levobunolol (INN); Levobunolol HCl; Levobunolol [INN:BAN]; Levobunololum; Levobunololum [INN-Latin]; W-7000A; l-Bunolol; Akbeta; Betagan; LEVOBUNOLOL; (-)-Bunolol; (S)-5-(3-(tert-Butylamino)-2-hydroxypropoxy)-3,4-dihydronaphthalen-1(2H)-one; 1(2H)-Naphthalenone, 5-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)-3,4-dihydro-, (S)-; 47141-42-4; 5-[(2S)-3-(tert-butylamino)-2-hydroxypropoxy]-3,4-dihydro-2H-naphthalen-1-one; CCRIS 4375; CHEBI:6438; G6317AOI7K; UNII-G6317AOI7K
|
| Cross-matching ID |
- PubChem CID
- 39468
- PubChem SID
-
10116
; 7979771
; 8147013
; 8176045
; 11112712
; 11112713
; 11466875
; 11467995
; 11486490
; 14800093
; 34705356
; 46507518
; 47216948
; 47440435
; 47810938
; 47810939
; 49698660
; 50064797
; 50100511
; 57312300
; 75377008
; 85787332
; 96024805
; 104331810
; 117598030
; 123099352
; 128233843
; 134223509
; 134337615
; 135002286
; 135650031
; 137004994
; 141191903
; 160964543
; 162172878
; 163418962
; 163851154
; 164789572
; 172860923
; 175268158
; 179150487
; 184531333
; 198967741
; 223554927
; 224987628
; 226412538
; 252351251
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00IUG
- Formula
- C17H25NO3
- Canonical SMILES
- CC(C)(C)NCC(COC1=CC=CC2=C1CCCC2=O)O
- InChI
- 1S/C17H25NO3/c1-17(2,3)18-10-12(19)11-21-16-9-5-6-13-14(16)7-4-8-15(13)20/h5-6,9,12,18-19H,4,7-8,10-11H2,1-3H3/t12-/m0/s1
- InChIKey
- IXHBTMCLRNMKHZ-LBPRGKRZSA-N
|