| General Information of Drug (ID:
DR0942) |
| Drug Name |
Levomepromazine
|
| Synonyms |
Levomepromazin; Levomepromazina; Levomepromazina [INN-Spanish]; Levomepromazine; Levomepromazinum; Levomepromazinum [INN-Latin]; Levopromazioni; Levoprome; Levotomin; METHOTRIMEPRAZINE; Methotrimeprazine (USP); Methotrimeprazine [USP]; Methoxytrimeprazine; Milezin; Minozinan; Neozine; Nirnamine; Nocinan; Nomizan; Nosinan; Nozinan; RP-7044; SK&F-5116; Veractil; 2-Methoxytrimeprazine; 60-99-1; 9G0LAW7ATQ; Bayer 1213; CHEBI:6838; CL 36467; CL 39743; EINECS 200-495-5; UNII-9G0LAW7ATQ; Arc-VI-C-5; Dedoran; Hirnamine; XP03
|
| Indication |
Insomnia
[ICD11: 7A00]
|
Approved
|
[1]
|
| Structure |
|
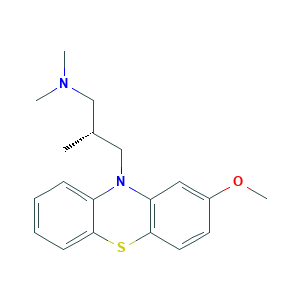 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
328.5 |
Topological Polar Surface Area |
41 |
| Heavy Atom Count |
23 |
Rotatable Bond Count |
5 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 72287
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09LZT
- Formula
- C19H24N2OS
- Canonical SMILES
- CC(CN1C2=CC=CC=C2SC3=C1C=C(C=C3)OC)CN(C)C
- InChI
- 1S/C19H24N2OS/c1-14(12-20(2)3)13-21-16-7-5-6-8-18(16)23-19-10-9-15(22-4)11-17(19)21/h5-11,14H,12-13H2,1-4H3/t14-/m1/s1
- InChIKey
- VRQVVMDWGGWHTJ-CQSZACIVSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


