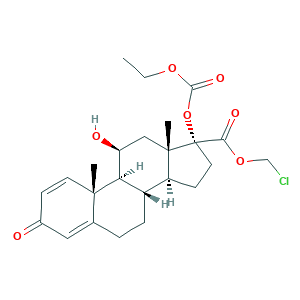
| Synonyms |
Locort; Loteflam; Lotemax; Lotemax (TN); Loteprednol etabonate; Loterox; P-5604; CDDD 5604; CDDD-5604; Inveltys; YEH1EZ96K6; 82034-46-6; Alrex; C24H31ClO7; CHEBI:31784; DSSTox_CID_26468; DSSTox_GSID_46468; DSSTox_RID_81641; HGP 1; HGP-1; UNII-YEH1EZ96K6; chloromethyl (8S,9S,10R,11S,13S,14S,17R)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-17-carboxylate; chloromethyl 17alpha-[(ethoxycarbonyl)oxy]-11beta-hydroxy-3-oxoandrosta-1,4-diene-17beta-carboxylate
|
| Cross-matching ID |
- PubChem CID
- 444025
- PubChem SID
-
7848752
; 10299468
; 12014581
; 14809424
; 14834133
; 36887139
; 46386647
; 49681758
; 53788349
; 56352909
; 57404592
; 71824934
; 74388043
; 92308346
; 103770848
; 104253297
; 104631159
; 109692941
; 124659028
; 124757418
; 124800193
; 125164222
; 126592942
; 127755782
; 134338307
; 135014568
; 135692220
; 137023315
; 140684370
; 144205772
; 152258757
; 160647602
; 160964214
; 162179036
; 164811824
; 170465195
; 172080414
; 175266502
; 176484705
; 178103663
; 179151237
; 184664942
; 226412422
; 249850601
; 252220094
; 252359498
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X6GN
- Formula
- C24H31ClO7
- Canonical SMILES
- CCOC(=O)OC1(CCC2C1(CC(C3C2CCC4=CC(=O)C=CC34C)O)C)C(=O)OCCl
- InChI
- 1S/C24H31ClO7/c1-4-30-21(29)32-24(20(28)31-13-25)10-8-17-16-6-5-14-11-15(26)7-9-22(14,2)19(16)18(27)12-23(17,24)3/h7,9,11,16-19,27H,4-6,8,10,12-13H2,1-3H3/t16-,17-,18-,19+,22-,23-,24-/m0/s1
- InChIKey
- DMKSVUSAATWOCU-HROMYWEYSA-N
|