| General Information of Drug (ID:
DR1440) |
| Drug Name |
Ropinirole hydrochloride
|
| Synonyms |
Ropinirole (hydrochloride); Ropinirole HCl; Ropinirole hydrochloride; Ropinirole hydrochloride [USAN:USP]; Ropinirol [INN-Spanish]; Ropinirole [INN:BAN]; Ropinirolum; Ropinirolum [INN-Latin]; Ropitor (TN); SK&F 101468; SKF 101468; UHSKFQJFRQCDBE-UHFFFAOYSA-N; UNII-030PYR8953; ropinirol; ropinirole; 030PYR8953; 2H-Indol-2-one, 4-(2-(dipropylamino)ethyl)-1,3-dihydro-; 4-(2-(dipropylamino)ethyl)indolin-2-one; 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one; 4-[2-(dipropylamino)ethyl]indolin-2-one; 91374-21-9; CHEBI:8888; CHEMBL589; DSSTox_CID_25195; DSSTox_GSID_45195; DSSTox_RID_80742; NCGC00015893-02; SK&F 101468-A; SK&F-101468A; SKF 101468 hydrochloride; SKF-101468A; UNII-D7ZD41RZI9; 1,3-Dihydro-4-(2-(dipropylamino)ethyl)-2H-indol-2-one monohydrochloride; 4-(2-(Dipropylamino)ethyl)-2-indolinone monohydrochloride; 4-(2-(Dipropylamino)ethyl)indolin-2-one hydrochloride; 4-[2-(dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one hydrochloride; 91374-20-8; D7ZD41RZI9; MFCD01754173; ReQuip XL
|
| Indication |
Parkinsonism
[ICD11: 8A00]
|
Approved
|
[1]
|
| Structure |
|
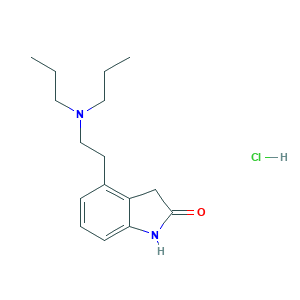 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
296.83 |
Topological Polar Surface Area |
32.299 |
| Heavy Atom Count |
20 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 68727
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0R9EQ
- Formula
- C16H25ClN2O
- Canonical SMILES
- CCCN(CCC)CCC1=C2CC(=O)NC2=CC=C1.Cl
- InChI
- 1S/C16H24N2O.ClH/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15;/h5-7H,3-4,8-12H2,1-2H3,(H,17,19);1H
- InChIKey
- XDXHAEQXIBQUEZ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


