| Synonyms |
Ruxolitinib (phosphate); Ruxolitinib phosphate; Ruxolitinib phosphate salt; Ruxolitinib phosphate(INCB018424); UNII-436LRU32H5; (R)-3-(4-(7H-Pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate; (betaR)-beta-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile phosphate; 1092939-17-7; 436LRU32H5; CHEBI:66917; INCB 018424 phosphate; J-501793; Jakafi; Jakavi
|
| Cross-matching ID |
- PubChem CID
- 25127112
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D8KQ
- Formula
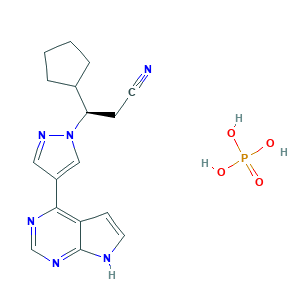
- C17H21N6O4P
- Canonical SMILES
- C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3.OP(=O)(O)O
- InChI
- 1S/C17H18N6.H3O4P/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16;1-5(2,3)4/h6,8-12,15H,1-5H2,(H,19,20,21);(H3,1,2,3,4)/t15-;/m1./s1
- InChIKey
- JFMWPOCYMYGEDM-XFULWGLBSA-N
|