| Synonyms |
Selinexor; Selinexor (KPT-330); Selinexor (USAN/INN); Selinexor [USAN:INN]; Tube706; Xpovio; (Z)-3-(3-(3,5-Bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-N'-(pyrazin-2-yl)acrylohydrazide; 1393477-72-9; 2-Propenoic acid, 3-(3-(3,5-bis(trifluoromethyl)phenyl)-1H-1,2,4-triazol-1-yl)-, 2-(2-pyrazinyl)hydrazide, (2Z)-; 2-Propenoic acid, 3-[3-[3,5-bis(trifluoromethyl)phenyl]-1H-1,2,4-triazol-1-yl]-, 2-(2-pyrazinyl)hydrazide, (2Z)-; KPT 330; KPT-330; KPT-330(Selinexor); KPT330; 31TZ62FO8F; UNII-31TZ62FO8F
|
| Cross-matching ID |
- PubChem CID
- 71481097
- PubChem SID
-
163453790
; 172650933
; 174009346
; 198946299
; 223471411
; 224765892
; 239654024
; 252472214
- CAS Number
-
- TTD Drug ID
- D00LNW
- Formula
- C17H11F6N7O
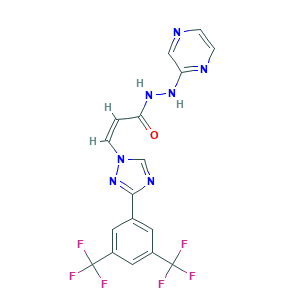
- Canonical SMILES
- C1=CN=C(C=N1)NNC(=O)C=CN2C=NC(=N2)C3=CC(=CC(=C3)C(F)(F)F)C(F)(F)F
- InChI
- 1S/C17H11F6N7O/c18-16(19,20)11-5-10(6-12(7-11)17(21,22)23)15-26-9-30(29-15)4-1-14(31)28-27-13-8-24-2-3-25-13/h1-9H,(H,25,27)(H,28,31)/b4-1-
- InChIKey
- DEVSOMFAQLZNKR-RJRFIUFISA-N
|