| Synonyms |
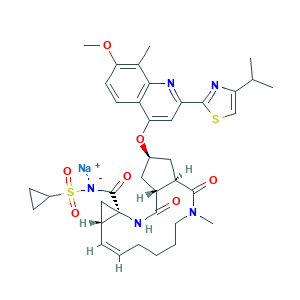
Simeprevir sodium; TMC435; TMC435350; CHEMBL3137358; 1241946-89-3; Q27251840; LLXQGDWGCCKOQP-MVZLLIIPSA-M; 9WS5RD66HZ; MFCD25563225; sodium;cyclopropylsulfonyl-[(1R,4R,6S,7Z,15R,17R)-17-[7-methoxy-8-methyl-2-(4-propan-2-yl-1,3-thiazol-2-yl)quinolin-4-yl]oxy-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.04,6]octadec-7-ene-4-carbonyl]azanide; (1R,4R,6R,7Z,15R,17R)-N-(cyclopropanesulfonyl)-17-({7-methoxy-8-methyl-2-[4-(propan-2-yl)-1,3-thiazol-2-yl]quinolin-4-yl}oxy)-13-methyl-2,14-dioxo-3,13-diazatricyclo[13.3.0.0^{4,6}]octadec-7-ene-4-carboxamide
|
| Cross-matching ID |
- PubChem CID
- 46866715
- CAS Number
-
- TTD Drug ID
- D00TLP
- Formula
- C38H46N5NaO7S2
- Canonical SMILES
- CC1=C(C=CC2=C1N=C(C=C2OC3CC4C(C3)C(=O)N(CCCCC=CC5CC5(NC4=O)C(=O)[N-]S(=O)(=O)C6CC6)C)C7=NC(=CS7)C(C)C)OC.[Na+]
- InChI
- 1S/C38H47N5O7S2.Na/c1-21(2)30-20-51-35(40-30)29-18-32(26-13-14-31(49-5)22(3)33(26)39-29)50-24-16-27-28(17-24)36(45)43(4)15-9-7-6-8-10-23-19-38(23,41-34(27)44)37(46)42-52(47,48)25-11-12-25;/h8,10,13-14,18,20-21,23-25,27-28H,6-7,9,11-12,15-17,19H2,1-5H3,(H2,41,42,44,46);/q;+1/p-1/b10-8-;/t23-,24-,27-,28-,38-;/m1./s1
- InChIKey
- LLXQGDWGCCKOQP-MVZLLIIPSA-M
|