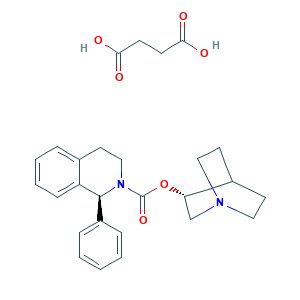
| Synonyms |
Solifenacin (Succinate); Solifenacin succinate; Solifenacin succinate [USAN]; Solifenacin (INN); Solifenacin [INN:BAN]; Soliten; UNII-A8910SQJ1U; [(8R)-1-azabicyclo[2.2.2]octan-8-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate; ((8r)-1-azabicyclo(2.2.2)octan-8-yl) (1s)-1-phenyl-3,4-dihydro-1h-isoQUINOLINE-2-carboxylate; (+)-Solifenacin; (3R)-1-azabicyclo[2.2.2]octan-3-yl (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate; 242478-37-1; A8910SQJ1U; AC1L4BM3; CHEMBL1734; NCGC00168778-01; SCHEMBL188493; SCHEMBL9971260; UNII-KKA5DLD701; Vesikur; YM 905; 1-Azabicyclo[2.2.2]octan-8-yl (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate butanedioic acid; 242478-38-2; Butanedioic acid, compd with (1S)-(3R)-1-azabicyclo(2.2.2)oct-3-yl 3,4-dihydro-1-phenyl-2(1H)-isoquinolinecarboxylate (1:1); KKA5DLD701
|
| Cross-matching ID |
- PubChem CID
- 216457
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L4YD
- Formula
- C27H32N2O6
- Canonical SMILES
- C1CN2CCC1C(C2)OC(=O)N3CCC4=CC=CC=C4C3C5=CC=CC=C5.C(CC(=O)O)C(=O)O
- InChI
- 1S/C23H26N2O2.C4H6O4/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19;5-3(6)1-2-4(7)8/h1-9,18,21-22H,10-16H2;1-2H2,(H,5,6)(H,7,8)/t21-,22-;/m0./s1
- InChIKey
- RXZMMZZRUPYENV-VROPFNGYSA-N
|