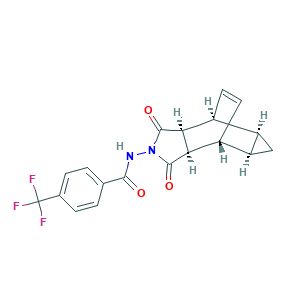
| Synonyms |
Tecovirimat; Tecovirimat [USAN:INN]; SIGA 246; SIGA-246; ST 246; ST-246; 816458-31-8; 869572-92-9; Benzamide, N-[(3aR,4R,4aR,5aS,6S,6aS)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl]-4-(trifluoromethyl)-, rel-; F925RR824R; N-((3aR,4R,4aR,5aS,6S,6aS)-1,3-Dioxo-3,3a,4,4a,5,5a,6,6a-octahydro-4,6-ethenocyclopropa(f)isoindol-2(1H)-yl)-4-(trifluoromethyl)benzamide; TPOXX; UNII-F925RR824R
|
| Cross-matching ID |
- PubChem CID
- 16124688
- CAS Number
-
- TTD Drug ID
- D08FOR
- Formula
- C19H15F3N2O3
- Canonical SMILES
- C1C2C1C3C=CC2C4C3C(=O)N(C4=O)NC(=O)C5=CC=C(C=C5)C(F)(F)F
- InChI
- 1S/C19H15F3N2O3/c20-19(21,22)9-3-1-8(2-4-9)16(25)23-24-17(26)14-10-5-6-11(13-7-12(10)13)15(14)18(24)27/h1-6,10-15H,7H2,(H,23,25)/t10-,11+,12+,13-,14-,15+
- InChIKey
- CSKDFZIMJXRJGH-VWLPUNTISA-N
|