Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1652) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Troleandomycin
|
|||||
| Synonyms |
Treolmicina; Triacetyl ester of oleandomycin; Triacetyloleandomycinum; Triolan; Troleandomicina [INN-Spanish]; Troleandomycin [USAN:INN]; Troleandomycine [INN-French]; Troleandomycinum [INN-Latin]; Aovine; Cyclamycin; J-016795; LS-98282; Matromicina; Matromycin T; Oleandocetin; Oleandomycin, triacetyl-; Prestwick_594; TROLEANDOMYCIN; Tao (VAN); Treis-Micina; Viamicina; WY 651; Wytrion; AC1L2AG5; AI3-50166; C41H67NO14; CTK8G3654; EINECS 220-392-9; GTPL10209; NSC 108166; T.A.O; TAO
|
|||||
| Indication | Acute upper respiratory infection [ICD11: CA07] | Approved | [1] | |||
| Structure |
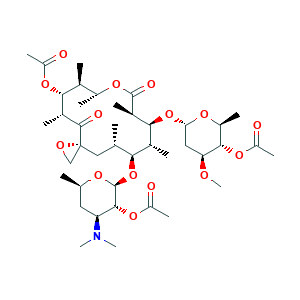 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 814 | Topological Polar Surface Area | 184 | ||
| Heavy Atom Count | 57 | Rotatable Bond Count | 12 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 16 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

