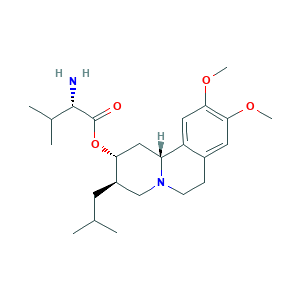
| Synonyms |
Valbenazine; Valbenazine [USAN:INN]; Ingrezza; NBI 98854; 1025504-45-3; 54K37P50KH; L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester; L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester;L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester; UNII-54K37P50KH
|
| Cross-matching ID |
- PubChem CID
- 24795069
- PubChem SID
-
49738821
; 57114321
; 163370736
; 164133227
; 198945028
; 224577868
; 240859006
; 246330347
; 252166894
- CAS Number
-
- TTD Drug ID
- D0IX1I
- Formula
- C24H38N2O4
- Canonical SMILES
- CC(C)CC1CN2CCC3=CC(=C(C=C3C2CC1OC(=O)C(C(C)C)N)OC)OC
- InChI
- 1S/C24H38N2O4/c1-14(2)9-17-13-26-8-7-16-10-21(28-5)22(29-6)11-18(16)19(26)12-20(17)30-24(27)23(25)15(3)4/h10-11,14-15,17,19-20,23H,7-9,12-13,25H2,1-6H3/t17-,19-,20-,23+/m1/s1
- InChIKey
- GEJDGVNQKABXKG-CFKGEZKQSA-N
|