Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1746) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Dicumarol
|
|||||
| Synonyms |
Dicumarol; Dicoumarol; Bishydroxycoumarin; Dicoumarin; melitoxin; Bis-hydroxycoumarin; Antitrombosin; Baracoumin; Dicoumal; Dicumarine; Acadyl; Acavyl; Dicuman; Trombosan; Dicumol; Dufalone; Kumoran; Temparin; Cumid; Cuma; Dicumaol R; 3,3'-Methylenebis(4-hydroxycoumarin); Bis(4-hydroxycoumarin-3-yl)methane; Anathrombase; Dicoumarolum; Apekumarol; Dicoumerol; Dicumarinum; Dicumarolum; Bis-3,3'-(4-hydroxycoumarinyl)methane; Di-(4-hydroxy-3-coumarinyl)methane; Dicumarolo [DCIT]; Dwukumarol [Polish]; Di-4-hydroxy-3,3'-methylenedicoumarin; Dicumarol [INN-Spanish]; Dicoumarolum [INN-Latin]; 3,3'-Methylen-bis(4-hydroxy-cumarin); 3,3'-Methylenebis(4-hydroxy-2H-1-benzopyran-2-one); Dicumarol [USAN]; 66-76-2
|
|||||
| Indication | Menorrhagia [ICD11: GA20] | Approved | [1] | |||
| Structure |
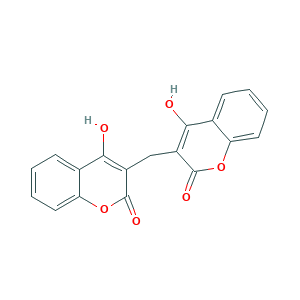 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 336.3 | Topological Polar Surface Area | 93.1 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

