| Synonyms |
Aristocort diacetate; Polcortolon; Tedarol; Aristocort Forte; Triamcinolone 16,21-diacetate; Orion; Aristocort Parenterals; Kenacort Diacetate Syrup; Aristocort diacetate forte; Aristocort Forte Parenteral; TRIAMCINOLONE DIACETATE; UNII-A73MM2Q32P; Kenacourt; Aristocort syrup; CHEBI:9669; 357RP; EINECS 200-669-0; Triamcinolone 16-alpha,21-di(acetate); A73MM2Q32P; 16alpha-Hydroxy-9alpha-fluoroprednisolone diacetate; 9-alpha-Fluoro-16-alpha-hydroxyprednisolone diacetate; NCGC00159320-03; delta1,9alpha-Fluoro-16alpha-hydroxyhydrocortisone diacetate; delta11,16alpha-Hydroxy-9alpha-fluorohydrocortisone diacetate; 16alpha,21-Diacetoxy-9alpha-fluoro-11beta,17alpha-dihydroxy-1,4-pregnadiene-3,20-dione; 9-Fluoro-11-beta,16-alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione 16,21-diacetate; 9-Fluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione 16,21-diacetate; DSSTox_CID_28639; DSSTox_RID_82909; DSSTox_GSID_48713; Pregna-1,4-diene-3,20-dione, 16,21-bis(acetyloxy)-9-fluoro-11,17-dihydroxy-, (11beta,16alpha)-; Polcartolone; CAS-67-78-7; Triamcinolone diacetate [JAN]; Triamcinolone diacetate [USP:JAN]; SCHEMBL12549; 67-78-7
|
| Cross-matching ID |
- PubChem CID
- 6216
- ChEBI ID
-
- CAS Number
-
- Formula
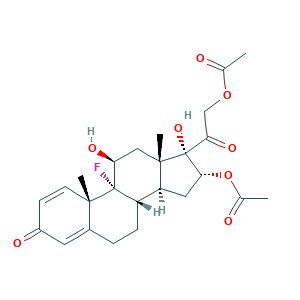
- C25H31FO8
- Canonical SMILES
- CC(=O)OCC(=O)C1(C(CC2C1(CC(C3(C2CCC4=CC(=O)C=CC43C)F)O)C)OC(=O)C)O
- InChI
- 1S/C25H31FO8/c1-13(27)33-12-20(31)25(32)21(34-14(2)28)10-18-17-6-5-15-9-16(29)7-8-22(15,3)24(17,26)19(30)11-23(18,25)4/h7-9,17-19,21,30,32H,5-6,10-12H2,1-4H3/t17-,18-,19-,21+,22-,23-,24-,25+/m0/s1
- InChIKey
- XGMPVBXKDAHORN-RBWIMXSLSA-N
|