| Cross-matching ID |
- PubChem CID
- 5281107
- PubChem SID
-
10410
; 7848072
; 8616516
; 11528742
; 14714977
; 14757783
; 14831187
; 26759741
; 39290018
; 47721666
; 50096685
; 50140476
; 53790000
; 57358018
; 71855033
; 103770793
; 109693717
; 113854564
; 126670553
; 127324039
; 127324040
; 127324041
; 134957119
; 135079074
; 135651481
; 135698305
; 137248797
; 142560574
; 143493351
; 144206531
; 152164533
; 152238015
; 152258777
; 160647628
; 162189015
; 163687121
; 164227093
; 165238070
; 175266149
; 176264149
; 184593516
; 184812060
; 210277996
; 223439572
; 223860398
; 226660079
; 251915651
; 251917001
; 252157093
; 252160414
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G5CF
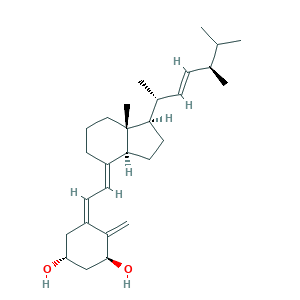
- Formula
- C28H44O2
- Canonical SMILES
- CC(C)C(C)C=CC(C)C1CCC2C1(CCCC2=CC=C3CC(CC(C3=C)O)O)C
- InChI
- 1S/C28H44O2/c1-18(2)19(3)9-10-20(4)25-13-14-26-22(8-7-15-28(25,26)6)11-12-23-16-24(29)17-27(30)21(23)5/h9-12,18-20,24-27,29-30H,5,7-8,13-17H2,1-4,6H3/b10-9+,22-11+,23-12-/t19-,20+,24+,25+,26-,27-,28+/m0/s1
- InChIKey
- HKXBNHCUPKIYDM-CGMHZMFXSA-N
|