| Synonyms |
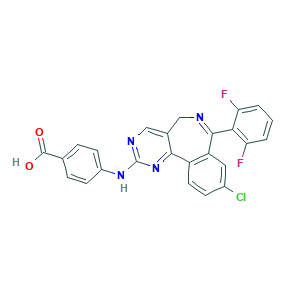
MLN 8054; MLN-8054; MLN8054; 4-((9-chloro-7-(2,6-difluorophenyl)-5h-pyrimido(5,4-d)(2)benzazepin-2-yl)amino)benzoic acid; 4-[[9-Chloro-7-(2,6-difluorophenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino]benzoic acid; 4-{[13-chloro-10-(2,6-difluorophenyl)-3,5,9-triazatricyclo[9.4.0.0^{2,7}]pentadeca-1(15),2(7),3,5,9,11,13-heptaen-4-yl]amino}benzoic acid; 4-{[9-chloro-7-(2,6-difluorophenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino}benzoic acid; 869363-13-3; BX854EHD63; CHEMBL259084; UNII-BX854EHD63
|
| Cross-matching ID |
- PubChem CID
- 11712649
- PubChem SID
-
16817521
; 23803070
; 50100109
; 75812624
; 85240458
; 87325745
; 92728686
; 103565670
; 103904597
; 117695998
; 123055394
; 124756998
; 125163803
; 131480681
; 134338844
; 134964414
; 135686180
; 135686181
; 135686202
; 135686203
; 136340201
; 136367298
; 136920349
; 137242800
; 137739931
; 152258105
; 152344040
; 160646944
; 162011816
; 162037433
; 162723440
; 163642777
; 164193990
; 164763083
; 170496031
; 174006403
; 174531092
; 177749042
; 178102323
; 180190865
; 186022471
; 198945325
; 215776778
; 223258872
; 223579841
; 223704701
; 225331045
; 226922686
; 242059849
; 244479327
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0A5MC
- Formula
- C25H15ClF2N4O2
- Canonical SMILES
- CCCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NN=N[N-]4)CO)Cl.[K+]
- InChI
- 1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32)
- InChIKey
- HHFBDROWDBDFBR-UHFFFAOYSA-N
|