| General Information of Drug (ID:
DR1865) |
| Drug Name |
BRN-0456976
|
| Synonyms |
Carfentanil [INN]; Carfentanila; Carfentanil; Carfentanila [INN-Spanish]; Carfentanila [Spanish]; Carfentanilum; Carfentanilum [INN-Latin]; Carfentanyl; Wildnil; CARFENTANIL; 4-((1-Oxopropyl)phenylamino)-1-(2-phenylethyl)-4-piperidinecarboxylic acid methyl ester; 59708-52-0; CHEBI:61084; Methyl 1-phenylethyl-4-(N-phenylpropionamido)isonipecotate; Methyl 4-(N-(1-oxopropyl)-N-phenylamino)-1-(2-phenylethyl)-4-piperidinecarboxylate; Methyl 4-(N-propionyl-N-phenylamino)-1-(2-phenylethyl)-4-piperidine-carboxylate; UNII-LA9DTA2L8F
|
| Indication |
Anaesthesia
[ICD11: 8E22]
|
Phase 2
|
[1]
|
| Structure |
|
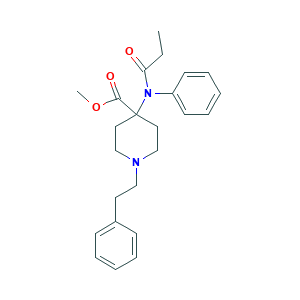 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
394.5 |
Topological Polar Surface Area |
49.8 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
8 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 62156
- PubChem SID
-
8187910
; 16847866
; 22395327
; 43119324
; 46505501
; 50015735
; 57314612
; 80840190
; 96024451
; 103224159
; 103924756
; 104222357
; 104325105
; 109614507
; 117553158
; 129780983
; 134222605
; 135020271
; 137129263
; 141935616
; 160964782
; 162630311
; 164837838
; 175443459
; 180100818
; 198942200
; 226488467
; 241102489
; 250116464
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D03PJU
- Formula
- C24H30N2O3
- Canonical SMILES
- CCC(=O)N(C1=CC=CC=C1)C2(CCN(CC2)CCC3=CC=CC=C3)C(=O)OC
- InChI
- 1S/C24H30N2O3/c1-3-22(27)26(21-12-8-5-9-13-21)24(23(28)29-2)15-18-25(19-16-24)17-14-20-10-6-4-7-11-20/h4-13H,3,14-19H2,1-2H3
- InChIKey
- YDSDEBIZUNNPOB-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


