| General Information of Drug (ID:
DR1891) |
| Drug Name |
YKP-509
|
| Synonyms |
Carisbamate; Carisbamate (USAN); Carisbamate [USAN:INN]; Comfyde; JNJ-10234094; OLBWFRRUHYQABZ-MRVPVSSYSA-N; P7725I9V3Z; RWJ 333369; RWJ-333369; SB18906; SCHEMBL729003; YKP-509; ZINC30691363; (+)-(2S)-2-(2-chlorophenyl)-2-hydroxyethyl carbamate; (S)-2-carbamoyloxy-1-o-chlorophenylethanol; (S)-Carisbamate; 194085-75-1; AC1OCFHA; API0009416; CHEBI:135966; CHEMBL2087003; D06573; DB12338; DTXSID70426076; FT-0664383; S-2-O-Carbamoyl-1-o-chlorophenyl-ethanol; UNII-P7725I9V3Z; X3698; [(2S)-2-(2-chlorophenyl)-2-hydroxyethyl] carbamate
|
| Indication |
Dyskinesia
[ICD11: 8A02]
|
Phase 3
|
[1]
|
| Structure |
|
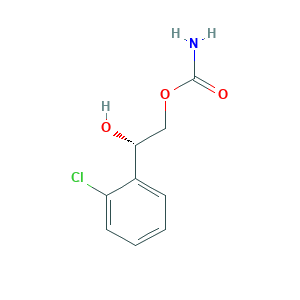 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
215.63 |
Topological Polar Surface Area |
72.6 |
| Heavy Atom Count |
14 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 6918474
- PubChem SID
-
12015336
; 14818877
; 15172026
; 17194912
; 43529844
; 47208229
; 57371948
; 79488142
; 114788030
; 128824565
; 134223530
; 134339232
; 135213416
; 140097639
; 160670319
; 164837840
; 184528548
; 198974092
; 198991959
; 223366886
; 225025837
; 227016974
; 247473631
; 252552117
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0S0FZ
- Formula
- C9H10ClNO3
- Canonical SMILES
- C1=CC=C(C(=C1)C(COC(=O)N)O)Cl
- InChI
- 1S/C9H10ClNO3/c10-7-4-2-1-3-6(7)8(12)5-14-9(11)13/h1-4,8,12H,5H2,(H2,11,13)/t8-/m1/s1
- InChIKey
- OLBWFRRUHYQABZ-MRVPVSSYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


