Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1905) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
LAS-17177
|
|||||
| Synonyms |
Blaston; Cidine; Cinitaprida; Cinitaprida [INN-Spanish]; Cinitapride; Cinitapride (INN); Cinitapride [INN]; Cinitapride hydrogen tartrate; Cinitapridum; Cinitapridum [INN-Latin]; Cinmove; Cintapro; Paxapride; Paxapride (TN); R8I97I2L24; SCHEMBL476454; cinitapride tartrate; (non-labelled)Cinitapride-d5; 4-amino-N-[1-(cyclohex-3-en-1-ylmethyl)piperidin-4-yl]-2-ethoxy-5-nitrobenzamide; 66564-14-5; AC1L2AM4; AC1Q1YMD; AKOS015909742; BCP04096; C21H30N4O4; CHEBI:135642; CHEMBL2104523; DB08810; SCHEMBL19235643; UNII-R8I97I2L24; cidin
|
|||||
| Indication | Functional nausea/vomiting [ICD11: DD90] | Phase 3 | [1] | |||
| Structure |
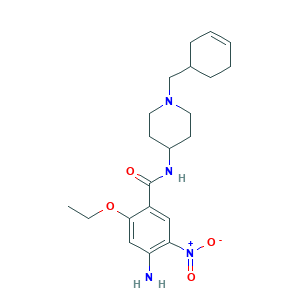 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 402.5 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

