| General Information of Drug (ID:
DR1923) |
| Drug Name |
Alprostadil
|
| Synonyms |
Alista; Alprostadil(Caverject); Alprostadilum; Alprox-TD; Befar (TN); Caverject; Femprox; Lipoprost; Liprostin; MR-256; Minprog; Prink (TN); Prostaglandin E1; Prostandin; Prostavasin; Prostin VR; Prostin VR Pediatric; Prostivas; Topiglan; Vasaprostan; Vitaros; alprostadil; l-Prostaglandin E1; (11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid; (13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate; 11alpha,15alpha-Dihydroxy-9-oxo-13-trans-prostenoic acid; 745-65-3; Befar; CHEMBL495; Edex; Muse; PGE-1; PGE1; Prink; UNII-F5TD010360
|
| Indication |
Erectile dysfunction
[ICD11: HA01]
|
Approved
|
[1]
|
| Structure |
|
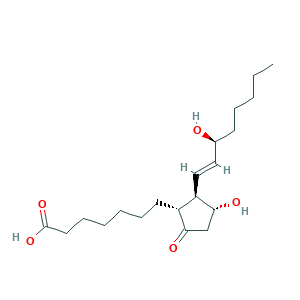 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
354.5 |
Topological Polar Surface Area |
94.8 |
| Heavy Atom Count |
25 |
Rotatable Bond Count |
13 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 5280723
- PubChem SID
-
7312
; 3139926
; 4266050
; 7847248
; 7978676
; 8143220
; 8616376
; 12013405
; 14901054
; 14901056
; 24887977
; 24890439
; 24898661
; 24898855
; 24898994
; 26719695
; 26752225
; 26752226
; 39289772
; 46386879
; 46386882
; 46387002
; 47216549
; 47515087
; 47515088
; 47810514
; 47959471
; 48110217
; 48184750
; 48415538
; 49681576
; 49699251
; 50104916
; 50104917
; 53790811
; 57357853
; 57654538
; 77812073
; 85789478
; 91702047
; 92126041
; 92308647
; 92309224
; 92309904
; 92712130
; 93166905
; 99300818
; 99302336
; 103171219
; 103914567
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0I4DQ
- Formula
- C20H34O5
- Canonical SMILES
- CCCCCC(C=CC1C(CC(=O)C1CCCCCCC(=O)O)O)O
- InChI
- 1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1
- InChIKey
- GMVPRGQOIOIIMI-DWKJAMRDSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


