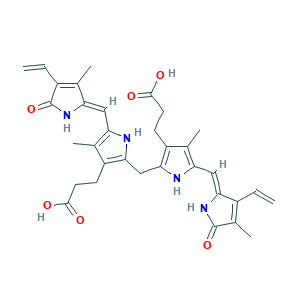
| Synonyms |
Bilirubin IX-alpha; Bilirubin IXalpha; Bilirubin, 99%; Disodium bilirubinate IXalpha; Hematoidin; Hemetoidin; PHEOPHYTIN; Principal bile pigment; RFM9X3LJ49; bilirubin; 21H-Biline-8,12-dipropanoic acid, 2,17-diethenyl-1,10,19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-; 635-65-4; 93891-87-3; AI3-23078; BPYKTIZUTYGOLE-IFADSCNNSA-N; BRN 0074376; Biline-8,12-dipropionic acid, 1,10,19,22,23,24-hexahydro-2,7,13,17-tetramethyl-1,19-dioxo-3,18-divinyl-; CHEBI:16990; EINECS 211-239-7; MFCD00005499; NSC 26685; UNII-RFM9X3LJ49
|
| Cross-matching ID |
- PubChem CID
- 5280352
- PubChem SID
-
3769
; 8144728
; 8616233
; 10319019
; 11537670
; 14935827
; 24848604
; 24891751
; 25630735
; 26612105
; 26681048
; 26749657
; 39289522
; 47348442
; 47868681
; 47942824
; 49746883
; 49834296
; 49893925
; 50096481
; 50096483
; 53789017
; 57357704
; 57647150
; 80330271
; 85245571
; 85248032
; 85248034
; 87563495
; 88266590
; 92718032
; 99455424
; 99455426
; 103619919
; 111677770
; 113853139
; 117636345
; 124636399
; 126522981
; 126628628
; 127269546
; 127269547
; 127269548
; 127269549
; 134977906
; 135252701
; 135253133
; 137144061
; 138836811
; 144072300
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O8ZV
- Formula
- C33H36N4O6
- Canonical SMILES
- CC1=C(NC(=C1CCC(=O)O)CC2=C(C(=C(N2)C=C3C(=C(C(=O)N3)C)C=C)C)CCC(=O)O)C=C4C(=C(C(=O)N4)C=C)C
- InChI
- 1S/C33H36N4O6/c1-7-20-19(6)32(42)37-27(20)14-25-18(5)23(10-12-31(40)41)29(35-25)15-28-22(9-11-30(38)39)17(4)24(34-28)13-26-16(3)21(8-2)33(43)36-26/h7-8,13-14,34-35H,1-2,9-12,15H2,3-6H3,(H,36,43)(H,37,42)(H,38,39)(H,40,41)/b26-13-,27-14-
- InChIKey
- BPYKTIZUTYGOLE-IFADSCNNSA-N
|