Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR2154) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
RTR-001007
|
|||||
| Synonyms |
Caffeic acid 2-phenylethyl ester; Caffeic acid phenethyl ester; Caffeic acid-phenethyl ester; Capeee; PHENETHYL CAFFEATE (CAPE); Phenethyl caffeate; Phenylethyl caffeate; SWUARLUWKZWEBQ-VQHVLOKHSA-N; caffeic acid phenylethyl ester; 100981-80-4; 104594-70-9; 115610-29-2; 2-Phenylethyl (2e)-3-(3,4-Dihydroxyphenyl; 2-phenylethyl 3-(3,4-dihydroxyphenyl)-2-propenoate; 2-phenylethyl caffeate; CAPE; CHEBI:8062; CHEMBL319244; Caffeic Acid Phenethyl Ester, Synthetic; G960R9S5SK; UNII-G960R9S5SK; phenethyl 3-(3,4-dihydroxyphenyl)acrylate
|
|||||
| Indication | Diabetes mellitus [ICD11: 5A10] | Phase 1 | [1] | |||
| Structure |
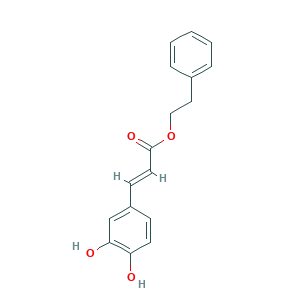 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 284.31 | Topological Polar Surface Area | 66.8 | ||
| Heavy Atom Count | 21 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

