| Synonyms |
Meclofenamate; Meclofenamic acid (USAN/INN); Meclofenamic acid [USAN:INN:BAN]; Meclomen (free acid); Meclophenamic acid; 2-((2,6-Dichloro-3-methylphenyl)amino)benzoic acid; 2-(2,6-Dichloro-3-methylphenyl)aminobenzoic acid; Acide meclofenamique; Acide meclofenamique [INN-French]; Acido meclofenamico; Acido meclofenamico [INN-Spanish]; Acidum meclofenamicum; Acidum meclofenamicum [INN-Latin]; Arquel; INF 4668; INF-4668; 2-(2,6-dichloro-3-methylanilino)benzoic acid; 2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid; CL 583; N-(2,6-Dichloro-3-methylphenyl)anthranilic acid; N-(2,6-Dichloro-m-tolyl)anthranilic acid; N-(3-Methyl-2,6-dichlorophenyl)anthranilic acid
|
| Cross-matching ID |
- PubChem CID
- 4037
- PubChem SID
-
9328
; 400193
; 840128
; 7457972
; 7849400
; 7979877
; 8152527
; 11335679
; 11360918
; 11363787
; 11366349
; 11368911
; 11371551
; 11373614
; 11377073
; 11461890
; 11466234
; 11467354
; 11484706
; 11485848
; 11488861
; 11490302
; 11491853
; 11494707
; 14800267
; 24424562
; 29223148
; 46507887
; 47365144
; 47440206
; 47588953
; 47662236
; 47959696
; 48110417
; 48110418
; 48334447
; 49846785
; 49870448
; 50103859
; 50264248
; 53788368
; 57322106
; 85173910
; 85788470
; 85789196
; 103173431
; 103828114
; 104305246
; 117467594
; 118313497
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08IFL
- Formula
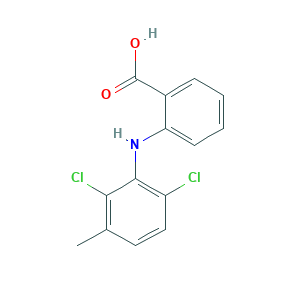
- C14H11Cl2NO2
- Canonical SMILES
- CC1=C(C(=C(C=C1)Cl)NC2=CC=CC=C2C(=O)O)Cl
- InChI
- 1S/C14H11Cl2NO2/c1-8-6-7-10(15)13(12(8)16)17-11-5-3-2-4-9(11)14(18)19/h2-7,17H,1H3,(H,18,19)
- InChIKey
- SBDNJUWAMKYJOX-UHFFFAOYSA-N
|