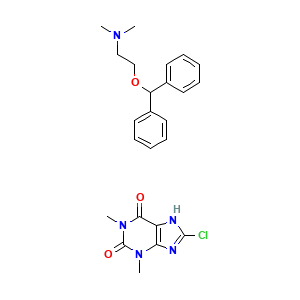
| Synonyms |
Gravamin (TN); Gravol (TN); Vertirosan (TN); Dimenhydrinate (JP15/USP/INN); 2-(diphenylmethoxy)-N,N-dimethylethanaminium 8-chloro-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurin-7-ide; 2-benzhydryloxyethyl(dimethyl)azanium; 8-chloro-1,3-dimethyl-2-oxopurin-6-olate; 8-chloro-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione-2-(diphenylmethoxy)-N,N-dimethylethanamine (1:1)
|
| Cross-matching ID |
- PubChem CID
- 10660
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U8UV
- Formula
- C24H28ClN5O3
- Canonical SMILES
- CN1C2=C(C(=O)N(C1=O)C)NC(=N2)Cl.CN(C)CCOC(C1=CC=CC=C1)C2=CC=CC=C2
- InChI
- InChI=1S/C17H21NO.C7H7ClN4O2/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16;1-11-4-3(9-6(8)10-4)5(13)12(2)7(11)14/h3-12,17H,13-14H2,1-2H3;1-2H3,(H,9,10)
- InChIKey
- NFLLKCVHYJRNRH-UHFFFAOYSA-N
|