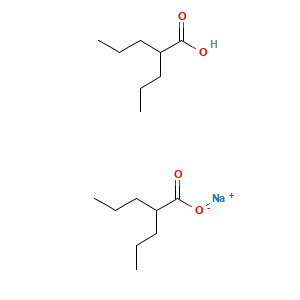
| Synonyms |
Delepsine; Depakote; Divalproate; Divalproex; Epilex; Epival; Sprinkle; Valcote; Valdisoval; Valparin; Depakote CP; Depakote ER; Depakote Sprinkle; Divalproex sodium [USAN]; Sodium divalproate; Sodium hydrogen divalproate; Valproate semisodique; Valproate semisodique [French]; Valproate semisodium; Valproato semisodico; Valproato semisodico [Spanish]; Valproatum seminatricum; Valproatum seminatricum [Latin]; Abbott 50711; Abbott-50711; Depacon (TN); Depakote (TN); Depakote ER (TN); Divalproex sodium (USP); Epival (TN); Ergenyl Chrono (TN); Valance (TN); Valproate semisodium (INN); Divalproex sodium, Depakote, Epival; Natrium hydrogen bis(2-propylvalerat); Sodium hydrogen bis(2-propylpentanoate); Sodium hydrogen bis(2-propylvalerate); Valproic acid semisodium salt (2:1); Zalkote. (TN); Sodium hydrogen bis(2-propylvalerate), oligomer; Sodium 2-propylpentanoate-2-propylpentanoic acid (1:1); Pentanoic acid, 2-propyl-, sodium salt (2:1); Pentanoic acid, 2-propyl-, sodium salt(2:1); 2-propylpentanoate
|